Whole plant cell wall characterization using solution-state 2D NMR
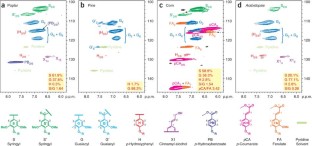
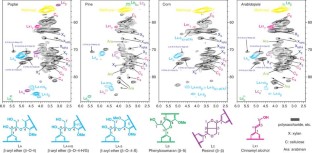
Recent advances in nuclear magnetic resonance (NMR) technology have made it possible to rapidly screen plant material and discern whole cell wall information without the need to deconstruct and fractionate the plant cell wall. This approach can be used to improve our understanding of the biology of cell wall structure and biosynthesis, and as a tool to select plant material for the most appropriate industrial applications. This is particularly true in an era when renewable materials are vital to the emerging bio-based economies. This protocol describes procedures for (i) the preparation and extraction of a biological plant tissue, (ii) solubilization strategies for plant material of varying composition and (iii) 2D NMR acquisition (for typically 15 min–5 h) and integration methods used to elucidate lignin subunit composition and lignin interunit linkage distribution, as well as cell wall polysaccharide profiling. Furthermore, we present data that demonstrate the utility of this new NMR whole cell wall characterization procedure with a variety of degradative methods traditionally used for cell wall compositional analysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Wood cellulose microfibrils have a 24-chain core–shell nanostructure in seed plants
Article 22 June 2023

Bundling of cellulose microfibrils in native and polyethylene glycol-containing wood cell walls revealed by small-angle neutron scattering
Article Open access 30 November 2020

Molecular architecture of softwood revealed by solid-state NMR
Article Open access 31 October 2019
References
- Weng, J.K. & Chapple, C. The origin and evolution of lignin biosynthesis. New Phytologist.187, 273–285 (2010). CASGoogle Scholar
- Vanholme, R., Morreel, K., Ralph, J. & Boerjan, W. Lignin biosynthesis and structure. Plant Physiol.153, 895–905 (2010). PubMedPubMed CentralCASGoogle Scholar
- Ralph, J. et al. Lignins: natural polymers from oxidative coupling of 4-hydroxyphenylpropanoids. Phytochem. Revs.3, 29–60 (2004). CASGoogle Scholar
- Boerjan, W., Ralph, J. & Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol.54, 519–549 (2003). PubMedCASGoogle Scholar
- Sarkanen, K.V. & Ludwig, C.H. Lignins, Occurrence, Formation, Structure and Reactions (Wiley-Interscience, 1971).
- Freudenberg, K. & Neish, A.C. Constitution and Biosynthesis of Lignin (Springer-Verlag, 1968).
- Ralph, J. & Landucci, L.L. NMR of lignins. in Lignin and Lignans; Advances in Chemistry (eds. Heitner, C., Dimmel, D.R. & Schmidt, J.A.) 137–234 (CRC Press, 2010).
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Revs.9, 65–83 (2010). CASGoogle Scholar
- Vanholme, R., Morreel, K., Ralph, J. & Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol.11, 278–285 (2008). PubMedCASGoogle Scholar
- Ralph, J. et al. in Recent Advances in Polyphenol Research Vol. 1 (eds. Daayf, F., El Hadrami, A., Adam, L. & Balance, G.M.) Ch. 2, 36–66 (Wiley-Blackwell Publishing, 2008). Google Scholar
- Ralph, J. Perturbing lignification. in The Compromised Wood Workshop 2007 (eds. Entwistle, K., Harris, P.J. & Walker, J.) 85–112 (Wood Technology Research Centre, University of Canterbury, 2007).
- Ralph, J. et al. Peroxidase-dependent cross-linking reactions of p-hydroxycinnamates in plant cell walls. Phytochem. Revs.3, 79–96 (2004). CASGoogle Scholar
- Ralph, J. et al. Cell wall cross-linking in grasses by ferulates and diferulates. et al. in Lignin and Lignan Biosynthesis, Vol. 697. (eds. Lewis, N.G. & Sarkanen, S.) 209–236 (American Chemical Society, 1998).
- Jung, H.G. & Allen, M.S. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Animal Sci.73, 2774–2790 (1995). CASGoogle Scholar
- Jung, H.G. Forage lignins and their effects on fiber digestibility. Agron. J.81, 33–38 (1989). CASGoogle Scholar
- Stewart, J.J., Kadla, J.F. & Mansfield, S.D. The influence of lignin chemistry and ultrastructure on the pulping efficiency of clonal aspen (Populus tremuloides Michx.) Holzforschung60, 111–122 (2006). CASGoogle Scholar
- Chen, F. & Dixon, R.A. Genetic manipulation of lignin biosynthesis to improve biomass characteristics for agro-industrial processes. In Vitro Cell. Dev. Biol.—Animal44, S28–S29 (2008). Google Scholar
- Li, X., Weng, J.K. & Chapple, C. Improvement of biomass through lignin modification. Plant J.54, 569–581 (2008). PubMedCASGoogle Scholar
- Chapple, C., Ladisch, M. & Meilan, R. Loosening lignin's grip on biofuel production. Nat. Biotechnol.25, 746–748 (2007). PubMedCASGoogle Scholar
- Bonawitz, N.D. & Chapple, C. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu. Rev. Genet.44, 337–363 (2010). PubMedCASGoogle Scholar
- Simmons, B.A., Loqué, D. & Ralph, J. Advances in modifying lignin for enhanced biofuel production. Curr. Opin. Plant Biol.13, 313–320 (2010). PubMedCASGoogle Scholar
- Jouanin, L. et al. Comparison of the consequences on lignin content and structure of COMT and CAD downregulation in poplar and Arabidobsis thaliana. in Plantation Forest Biotechnology in the 21st Century (eds. Walter, C. & Carson, M.) 219–229 (Research Signpost, 2004).
- Boerjan, W. et al. in Molecular Breeding of Woody Plants, Vol. Progress in Biotechnology Series, Vol. 18 (eds. Morohoshi, N. & Komamine, A.), Ch. 23, 187–194 (Elsevier Science, 2001).
- Mansfield, S.D. Solutions for dissolution-engineering cell walls for deconstruction. Curr. Opin. Biotechnol.20, 286–294 (2009). PubMedCASGoogle Scholar
- Lin, S.Y. & Dence, C.W. Methods in Lignin Chemistry (Springer-Verlag, 1992).
- Lapierre, C. Application of new methods for the investigation of lignin structure. in Forage Cell Wall Structure and Digestibility (eds. Jung, H.G., Buxton, D.R., Hatfield, R.D. & Ralph, J.) 133–166 (American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, 1993).
- Rolando, C., Monties, B. & Lapierre, C. Thioacidolysis. in Methods in Lignin Chemistry (eds. Dence, C.W. & Lin, S.Y.) 334–349 (Springer-Verlag, 1992).
- Robinson, A.R. & Mansfield, S.D. Rapid analysis of poplar lignin monomer composition by a streamlined thioacidolysis procedure and near-infrared reflectance-based prediction modeling. Plant J.58, 706–714 (2009). PubMedCASGoogle Scholar
- Yamamura, M., Hattori, T., Suzuki, S., Shibata, D. & Umezawa, T. Microscale alkaline nitrobenzene oxidation method for high-throughput determination of lignin aromatic components. Plant Biotechnol.27, 305–310 (2010). CASGoogle Scholar
- Villar, J.C., Caperos, A. & GarciaOchoa, F. Oxidation of hardwood kraft-lignin to phenolic derivatives. Nitrobenzene and copper oxide as oxidants. J. Wood Chem. Technol.17, 259–285 (1997). CASGoogle Scholar
- Lu, F. & Ralph, J. Efficient ether cleavage in lignins: the derivatization followed by reductive cleavage procedure as a basis for new analytical methods. in Lignin and Lignan Biosynthesis (eds. Lewis, N.G. & Sarkanen, S.) 294–322 (American Chemical Society, 1998).
- Lu, F. & Ralph, J. Derivatization followed by reductive cleavage (DFRC method), a new method for lignin analysis: protocol for analysis of DFRC monomers. J. Agr. Food Chem.45, 2590–2592 (1997). CASGoogle Scholar
- Lu, F. & Ralph, J. The DFRC method for lignin analysis. Part 1. A new method for β-aryl ether cleavage: lignin model studies. J. Agr. Food Chem.45, 4655–4660 (1997). CASGoogle Scholar
- Morrison, I.M. Improvements in the acetyl bromide technique to determine lignin and digestibility and its application to legumes. J. Sci. Food Agr.23, 1463–1469 (1972). CASGoogle Scholar
- Morrison, I.M. Semimicro method for the determination of lignin and its use in predicting the digestibility of forage crops. J. Sci. Food Agr.23, 455–463 (1972). CASGoogle Scholar
- Fukushima, R.S. & Hatfield, R. Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J. Agr. Food Chem.52, 3713–3720 (2004). CASGoogle Scholar
- Fukushima, R.S. & Hatfield, R.D. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agr. Food Chem.49, 3133–3139 (2001). CASGoogle Scholar
- Yelle, D.J., Wei, D., Ralph, J. & Hammel, K.E. Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia placenta. Appl. Environ. Microbiol.13, 1091–1100 (2011). CASGoogle Scholar
- Rencoret, J. et al. Lignin composition and structure in young versus adult Eucalyptus globulus plants. Plant Physiol.155, 667–682 (2011). PubMedCASGoogle Scholar
- Lu, F. & Ralph, J. Solution-state NMR of lignocellulosic biomass. J. Biobased Mater. Bio.5, 169–180 (2011). CASGoogle Scholar
- Kim, H. & Ralph, J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6/pyridine-d5 . Org. Biomol. Chem.8, 576–591 (2010). PubMedCASGoogle Scholar
- Hedenström, M. et al. Identification of lignin and polysaccharide modifications in Populus pood by chemometric analysis of 2D NMR spectra from dissolved cell walls. Mol. Plant2, 933–942 (2009). PubMedGoogle Scholar
- Yelle, D.J., Ralph, J. & Frihart, C.R. Characterization of non-derivatized plant cell walls using high-resolution solution-state NMR spectroscopy. Magn. Reson. Chem.46, 508–517 (2008). PubMedPubMed CentralCASGoogle Scholar
- Kim, H., Ralph, J. & Akiyama, T. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d6 . BioEnergy Res.1, 56–66 (2008). Google Scholar
- Ralph, J. & Lu, F. Cryoprobe 3D NMR of acetylated ball-milled pine cell walls. Org. Biomol. Chem.2, 2714–2715 (2004). PubMedCASGoogle Scholar
- Lu, F. & Ralph, J. Non-degradative dissolution and acetylation of ball-milled plant cell walls; high-resolution solution-state NMR. Plant J.35, 535–544 (2003). PubMedCASGoogle Scholar
- Ralph, J. et al. Solution-state NMR of lignins. in Advances in Lignocellulosics Characterization (ed. Argyropoulos, D.S.) 55–108 (TAPPI Press, 1999).
- Bradley, S.A. & Krishnamurthy, K. A modified CRISIS-HSQC for band-selective IMPRESS. Magn. Reson. Chem.43, 117–123 (2005). PubMedCASGoogle Scholar
- Boyer, R.D., Johnson, R. & Krishnamurthy, K. Compensation of refocusing inefficiency with synchronized inversion sweep (CRISIS) in multiplicity-edited HSQC. J. Magn. Reson.165, 253–259 (2003). PubMedCASGoogle Scholar
- Zhang, S.M., Wu, J. & Gorenstein, D.G. 'Double-WURST' decoupling for N-15- and C-13-double-labeled proteins in a high magnetic field. J. Magn. Reson. Series A123, 181–187 (1996). CASGoogle Scholar
- Kupce, E. & Freeman, R. Compensation for spin-spin coupling effects during adiabatic pulses. J. Magn. Reson.127, 36–48 (1997). CASGoogle Scholar
- Ralph, S.A., Landucci, L.L. & Ralph, J. NMR Database of Lignin and Cell Wall Model Compounds. http://ars.usda.gov/Services/docs.htm?docid=10491 (2004).
- Brennan, M., McLean, J.P., Altaner, C., Ralph, J. & Harris, P.J. Cellulose microfibril angles and cell-wall polymers in different wood types of Pinus radiata. Cellulose19, 1385–1404 (2012). CASGoogle Scholar
- Weng, J.-K., Akiyama, T., Ralph, J., Golden, B.L. & Chapple, C. Independent recruitment of an O-methyltransferase for syringyl lignin biosynthesis in Selaginella moellendorffii. Plant Cell23, 2708–2724 (2011). PubMedPubMed CentralCASGoogle Scholar
- Weng, J.-K. et al. Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. Plant Cell22, 1033–1045 (2010). PubMedPubMed CentralCASGoogle Scholar
- Vanholme, R. et al. Engineering traditional monolignols out of lignins by concomitant up-regulation F5H1 and down-regulation of COMT in Arabidopsis. Plant J.64, 885–897 (2010). PubMedCASGoogle Scholar
- Stewart, J.J., Akiyama, T., Chapple, C.C.S., Ralph, J. & Mansfield, S.D. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol.150, 621–635 (2009). PubMedPubMed CentralCASGoogle Scholar
- Ralph, J. et al. Identification of the structure and origin of a thioacidolysis marker compound for ferulic acid incorporation into angiosperm lignins (and an indicator for cinnamoyl-CoA reductase deficiency). Plant J.53, 368–379 (2008). PubMedCASGoogle Scholar
- Leplé, J.-C. et al. Downregulation of cinnamoyl coenzyme A reductase in poplar; multiple-level phenotyping reveals effects on cell wall polymer metabolism and structure. Plant Cell19, 3669–3691 (2007). PubMedPubMed CentralGoogle Scholar
- Ralph, J. et al. Effects of coumarate-3-hydroxylase downregulation on lignin structure. J. Biol. Chem.281, 8843–8853 (2006). PubMedCASGoogle Scholar
- Bunzel, M. & Ralph, J. NMR characterization of lignins isolated from fruit and vegetable insoluble dietary fiber. J. Agr. Food Chem.54, 8352–8361 (2006). CASGoogle Scholar
- Marita, J.M., Vermerris, W., Ralph, J. & Hatfield, R.D. Variations in the cell wall composition of maize brown midrib mutants. J. Agr. Food Chem.51, 1313–1321 (2003). CASGoogle Scholar
- Goujon, T. et al. A new Arabidopsis thaliana mutant deficient in the expression of O-methyltransferase impacts lignins and sinapoyl esters. Plant Mol. Biol.51, 973–989 (2003). PubMedCASGoogle Scholar
- Ralph, J. et al. Elucidation of new structures in lignins of CAD- and COMT-deficient plants by NMR. Phytochem.57, 993–1003 (2001). CASGoogle Scholar
- Marita, J., Ralph, J., Hatfield, R.D. & Chapple, C. NMR characterization of lignins in Arabidopsis altered in the activity of ferulate-5-hydroxylase. Proc. Natl. Acad. Sci. USA96, 12328–12332 (1999). PubMedPubMed CentralCASGoogle Scholar
- Hu, W.-J. et al. Repression of lignin biosynthesis in transgenic trees promotes cellulose accumulation and growth. Nat. Biotechnol.17, 808–812 (1999). PubMedCASGoogle Scholar
- Ralph, J., Akiyama, T., Coleman, H.D. & Mansfield, S.D. Effects on lignin structure of coumarate 3-hydroxylase downregulation in Poplar. BioEnergy Research, published online, doi:10.1007/s12155-012-9218-y (24 May 2012).
- Wagner, A. et al. Exploring lignification in conifers by silencing hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase in Pinus radiata. Proc. Natl. Acad. Sci. USA104, 11856–11861 (2007). PubMedPubMed CentralCASGoogle Scholar
- Zhang, L.M. & Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem.45, 37–45 (2007). PubMedCASGoogle Scholar
- Heikkinen, S., Toikka, M.M., Karhunen, P.T. & Kilpeläinen, I.A. Quantitative 2D HSQC (Q-HSQC) via suppression of J-dependence of polarization transfer in NMR spectroscopy: application to wood lignin. J. Am. Chem. Soc.125, 4362–4367 (2003). PubMedCASGoogle Scholar
- Hatfield, R.D., Marita, J.M. & Frost, K. Characterization of p-coumarate accumulation, p-coumaroyl transferase, and cell wall changes during the development of corn stems. J. Sci. Food Agr.88, 2529–2537 (2008). CASGoogle Scholar
- Hatfield, R.D. et al. Grass lignin acylation: p-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta229, 1253–1267 (2009). PubMedCASGoogle Scholar
Acknowledgements
We gratefully acknowledge funding from the Natural Sciences and Engineering Research Council of Canada′s Discovery Program held by S.D.M.; H.K., F.L., S.D.M. and J.R. were funded in part by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494).
Author information
Authors and Affiliations
- Department of Wood Science, University of British Columbia, Vancouver, Canada Shawn D Mansfield
- US Department of Energy (DOE) Great Lakes Bioenergy Research Center and Wisconsin Bioenergy Initiative, University of Wisconsin, Madison, Wisconsin, USA Hoon Kim, Fachuang Lu & John Ralph
- Shawn D Mansfield







